Egg Hatch
Factors maintaining the highly variable timing of egg hatch in Aedes and Ochlerotatus mosquitoes.
As many Aedes and Ochlerotatus mosquitoes do, O. triseriatus hatches from eggs laid in clusters on the sides of its larval habitat, usually just above the water line. Embryogenesis proceeds without interruption within a few days after oviposition, but the fully-formed embryos will not hatch until inundation and an ensuing drop in oxygen concentration have occurred. A portion of the eggs remain dormant until the water level has risen and receded repeatedly. Eggs deposited in late summer enter a photoperiod-induced diapause, and will not hatch until the following spring at the earliest. Some of this variation may stem from variable strength of stimulus or conditioning differences resulting from position effects within treeholes. Various lines of evidence point toward genetic basis for some of the variation that eggs exhibit. Gillett detected a heritable difference in the degree of variation in hatching times of two African strains of A. aegypti. Several other studies have detected differences in hatch rates between sympatric strains, Means et al. found a wide disparity in hatching responses of eggs from a treehole and a tire population of O. triseriatus, with the tire population showing much earlier and more synchronous hatching.
Hatch inhibition, along with a tendency for some eggs to refrain from hatching even in the absence of larval inhibitors, has several consequences for population dynamics. Edgerly and Livdahl (1992) manipulated the pattern of recruitment into larval environments and found that staggered entry into larval habitats, generated either by natural variability in hatch time or artificially by making weekly additions of newly hatched larvae, resulted in improved overall performance of experimental cohorts relative to synchronous entry, perhaps due to resource or spatial partitioning among larvae of different developmental stages. This interaction provides one of several possible environmental factors that affect the timing of egg hatch. However, Aedes and Ochlerotatus species display variability in hatch time even when efforts are made to minimize environmental variance, and that variability can be considerable (Livdahl and Koenekoop 1985). This variation presents some intriguing questions and some challenging conceptual issues.
Inheritance of life cycle delays. Variable timing of release from dormant stages is fairly common among invertebrates occupying temporary aquatic habitats (e.g. rotifers, copepods, mosquitoes), as well as many annual plants and spore-producing fungi and bacteria. Domesticated annual plants typically lack germination polymorphisms due to incidental selection against delayed seeds during domestication. Established laboratory colonies of Aedes and Ochlerotatus mosquitoes may also lack substantial hatch time variation for similar reasons: mass rearing typically entails hatching large numbers of eggs, harvesting the larvae, and discarding the eggs. Genotypes conveying either delayed hatch among individual eggs or highly variable hatch in the broods of females will be lost unless a concerted effort is made to retain unhatched and viable eggs. We might expect that a collection of tires at a landfill could have similar effects on the resident mosquito population: new tires are added to the population as old ones are made unavailable, and the only individuals that persist within those populations are those that hatch early and relatively synchronously. This may account for the high hatch rates on first immersion obtained in our lab for A. albopictus and A. aegypti (Edgerly et al. 1993) in the absence of larvae, as well as the difference between hatch rates of a population freshly established from treehole-collected larvae and tire-collected larvae in O. triseriatus, which resembled the hatch rate of an established laboratory strain (Means et al., Mosquito News, 1977).
Perhaps because the variability is not apparent to investigators working on lab strains, hatch time variation has not been studied extensively in Aedes and Ochlerotatus mosquitoes in recent years. Despite this neglect, the importance of variable hatch time to distributions in Aedes and Ochlerotatus mosquitoes is clear, and this feature may account for much of their success in exploiting ephemeral habitats. Aedes aegypti would likely still be an African treehole species had some of their eggs not refrained from hatching immediately in water storage vessels aboard slave ships (Gillett 1972). Aedes albopictus succeeded in colonizing North America in the mid-1980’s by virtue of some variation in hatch time: if all eggs had hatched, no individuals would have remained in shipments of used tires after lengthy trips across the Pacific from northern Japan, through the Panama Canal and Caribbean, to ports in Brazil and the southeastern U.S.
Evolutionary considerations. More attention has been paid to the evolution of plant seed germination rates and seed banks, with the supposition that seed germination can be described by a constant germination fraction: with a given stimulus, that fraction of seeds will germinate without regard to age. Rees (1994, American Naturalist) simulated this situation, and found that by enabling seeds to detect the presence of established plants (and to greatly reduce their germination rates) greatly increased the optimal basic germination fraction. Extending this conclusion to mosquitoes, we may expect that populations with higher basic hatching fractions may also possess greater sensitivity to larvae. This extension seems risky, but Rees’ study appears to be the only theoretical examination of these two phenomena to date. An alternative, intuitive expectation is also possible: dry habitats may be marked by longer periods between hatch stimuli; larvae are more likely to be absent when each stimulus occurs, either due to previous drought or emergence; therefore, the selective importance of refraining from hatching into environments is reduced, and the eggs may evolve a loss of sensitivity to larvae. This scenario suggests that hatch sensitivity should be inversely related to hatching fraction, but no formal framework has been developed to identify the necessary conditions.
Although it seems doubtful that all of these life cycle polymorphisms have arisen in response to the same sort of selective agent, “environmental uncertainty” and the “prevention of extinction” receive frequent reference. “Environmental uncertainty” could involve unpredictability of predator abundance, resource availability, competing species, or physical aspects of the habitat. The “prevention of extinction” in an uncertain environment may be a consequence of a life cycle polymorphism, but the evolution of such phenomena may result from a variety of alternative mechanisms. The intuitive explanation for variable dormancy, that the risk of a catastrophe is spread among individuals that hatch or germinate at variable times, has some theoretical backing from a variety of perspectives (Livdahl 1979, and other studies). For treehole mosquitoes, I hypothesize that hatch time variation has evolved in response to the likelihood that the larval habitat will dessicate, and our next project will conduct comparisons of egg hatch rates from 23 populations occupying a wide variety of precipitation regimes.
Theoretical snarls, and some possible resolutions. The conceptual difficulty associated with variable hatch or germination times concerns two different levels at which fitness can be viewed. A parent could impose variation of hatching or germinating on her offspring, as appears to occur in many plant species. In a situation of uncertain juvenile survivorship, a bet-hedging strategy of this sort can contribute to a parent’s fitness by minimizing the likelihood that all her offspring will be eliminated by a single drought or other catastrophe. However, a potential conflict between the fitness of parents and their offspring could arise: genes promoting the production of offspring with variable hatching or germinating times should be favored, but so should genes that promote early reproduction. An individual egg or seed has no guarantee that an extended dormant period will result in a higher survival probability, and a cost in fitness is imposed during periods of population growth by the extended generation time for the delayed individuals. This cost conflicts with benefits for parental fitness when females impose such delays on their offspring. The parent-offspring conflict can be removed by some form of compensation for larvae that delay their hatch. Although the precise mechanisms for this remain unclear, we have evidence for a substantial amount of structure within a population of O. triseriatusthat suggests that this sort of compensation occurs: late hatching larvae develop more quickly into larger adults, survive at higher rates, and are less sensitive to crowding than early-hatching larvae (Livdahl and Koenekoop 1985). Two potential mechanisms for this organization seem possible: structure may be maintained by the presence of particular sets of alleles at different loci that work well in combination (coadaptation); or loci that influence egg hatch may have concomitant effects on larval success (pleiotropy). We have theoretical reasons to expect the evolution of tightened linkage among loci that undergo simultaneous selection within certain limitations. Coadaptation may be detected when crosses between populations result in disruption of those combinations. Alternatively, pleiotropy may also account for organization of life history traits.
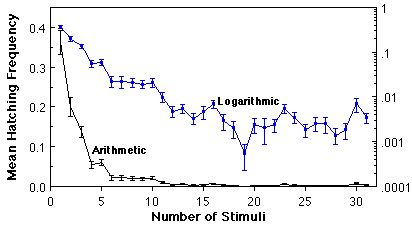
Variation in O. triseriatus hatching time and the associated structure of other life history traits. We have investigated variation in time required to hatch by subjecting eggs produced in the laboratory by field-captured females to successive weekly 24h stimuli (immersion in a brewer’s yeast suspension), interrupted by a 6 d period of damp storage (Livdahl and Koenekoop 1985). This procedure resulted in more than a 30-fold range of hatch times (above), with bursts of hatching occurring periodically quite late in the sequence. By rearing the larvae that hatched at different times under a spectrum of food and density conditions, it was possible to test for associations between hatching time and other life history features (development time, sex, adult size, survivorship), including the response of those life history features to crowding. A distinct organization of life history traits emerged from this analysis: larvae that hatch relatively late displayed superior overall survival, larger adult size, faster larval development, and a higher fraction of adult females (below, A-D). An aggregate measure of all of these features indicated a positive correlation between hatch time and per capita success (below, E), and reduced sensitivity to competition as hatch time increases (below, F).
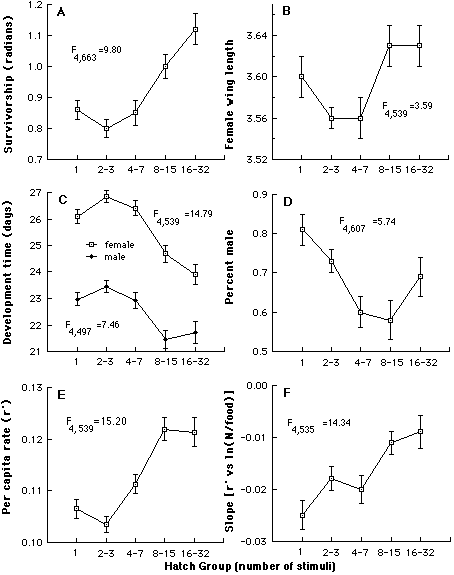
Summary of the organization of life history traits according to hatching time, compared by analysis of covariance. In (A-E, adjusted mean values ± S.E. of each group are shown. Tests for differences among the relative elevations of regression lines are summarized by F statistics, all of which are significant (p<0.01 in all cases). E shows a composite measure of success, and F shows the relative sensitivity of r' to density per unit food, in which more negative slopes indicate more sensitivity.