Kemeh MM and Lazo ND. Highly Toxic Aβ Begets More Aβ. Neural Regeneration Research 2024, 19(9), 1871-1872. This work was supported by a grant from the National Institute on Aging (R15AG055043) and by the Lise Ann and Leo E. Beavers II endowment to Clark University.
Kemeh MM and Lazo ND. Modulation of the Activity of the Insulin-degrading Enzyme by Aβ Peptides. ACS Chemical Neuroscience 2023, 14, 2935-2943. This work was supported by a grant from the National Institute on Aging (R15AG055043) and by the Lise Ann and Leo E. Beavers II endowment to Clark University.
Zheng Q, Lee B, Kebede MT, Ivancic VA, Kemeh MM, Lemos Brito H, Spratt DE and Lazo ND. Exchange Broadening Underlies the Enhancement of IDE-Dependent Degradation of Insulin by Anionic Membranes. ACS Omega 2022, 7, 24757-24765. This work was supported by a grant from the National Institute on Aging (R15AG055043).
Zheng Q, Kebede MT, Lee B, Krasinski CA, Islam S, Wurfl LA, Kemeh MM, Ivancic VA, Jakobsche CE, Spratt DE and Lazo ND. Differential Effects of Polyphenols on Insulin Proteolysis by the Insulin-degrading Enzyme. Antioxidants 2021, 10(1342), 1-11. This work was supported by a grant from the National Institute on Aging (R15AG055043).
Bocach DB, Jones KL, Bell JM, Zheng Q, Lazo ND, Smith-Carpenter JE, Alper BJ. Ghrelin Proteolysis by Insulin-degrading Enzyme. bioRxiv
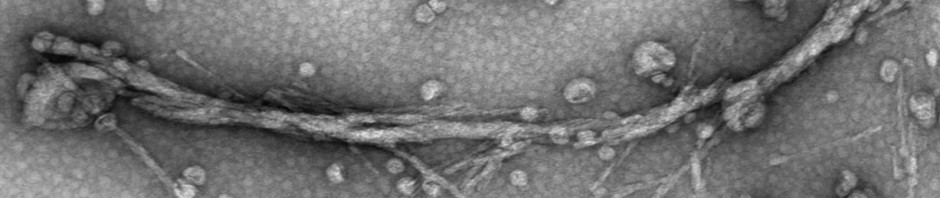
Zheng Q, Carty SN and Lazo ND. Helix Dipole and Membrane Electrostatics Delineate Conformational Transitions in the Self-Assembly of Amyloidogenic Peptides. Langmuir 2020, 86, 8389-8397. This work was supported by a grant from the National Institute on Aging (R15AG055043) and by the Lise Ann and Leo E. Beavers II endowment to Clark University.
DOI: 10.1021/acs.langmuir.0c00723
Zheng Q, Kebede MK, Kemeh MM, Islam S, Lee B, Bleck SD, Wurfl LA and Lazo ND. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules 2019, 24 (2316), 1-20. This work was supported by a grant from the National Institute on Aging (R15AG055043).
DOI: 10.3390/molecules24122316
Ivancic VA, Krasinski CA, Zheng Q, Meservier RJ, Spratt DE and Lazo ND. Enzyme kinetics from circular dichroism of insulin reveals mechanistic insights into the regulation of insulin-degrading enzyme. Bioscience Reports 2018, 38(6), 1-10. This work was supported by a grant from the National Institute on Aging (R15AG055043).
PMID: 30305381 PMCID: PMC6239264 DOI: 10.1042/BSR20181416
Krasinski CA, Ivancic VA, Zheng Q, Spratt DE and Lazo ND. Resveratrol sustains insulin-degrading enzyme activity towards Aß42. ACS Omega 2018, 3(10), 13275-13282. This work was supported by a grant from the National Institute on Aging (R15AG055043).
PMID: 30411033 PMCID: PMC6210067 DOI: 10.1021/acsomega.8b01913
Krasinski CA, Zheng Q, Ivancic VA, Spratt DE and Lazo ND. The longest amyloid-ß precursor protein intracellular domain produced with Aß42 forms ß-sheet-containing monomers that self-assemble and are proteolyzed by insulin-degrading enzyme. ACS Chemical Neuroscience. 2018, 9, 2892-2897. This work was supported by a grant from the National Institute on Aging (R15AG055043).
PMID: 30067897 DOI: 10.1021/acschemneuro.8b00305
Zheng Q and Lazo ND. Mechanistic studies of the inhibition of insulin fibril formation by rosmarinic acid. Journal of Physical Chemistry B 2018, 122, 2323-2331. This work was supported in part by a grant from the Lise Ann and Leo E. Beavers II endowment to Clark University.
PMID: 29401384 DOI: 10.1021/acs.jpcb.8b00689
Fontaine DFA, Ivancic VA, Reardon MB, Lazo ND, Jakobsche CE. A versatile platform for adding functional properties to amyloid fibrils. Organic Biomolecular Chemistry 2017, 15, 8023-8027.
PMID: 28930349 DOI: 10.1039/c7ob02042b
Ivancic VA, Ekayanake O and Lazo ND. Binding mode of thioflavin T on the surface of amyloid fibrils. ChemPhysChem 2016, 17, 2461-2464.
PMID: 27165642 DOI: 10.1002/cphc.201600246
Selmani V, Robbins KJ, Ivancic VA and Lazo ND. K114 (trans, trans)-bromo-2,5-bis (4-hydroxystyryl)benzene is an efficient detector of cationic amyloid fibrils. Protein Science 2015, 24, 420-425.
PMID: 25524064 PMCID: PMC4353367 DOI: 10.1002/pro.2620
Robbins KJ, Liu G, Selmani V and Lazo ND. Conformational analysis of thioflavin T bound to the surface of amyloid fibrils. Langmuir 2012, 28, 16490-16495.
PMID: 23151310 DOI: 10.1021/la303677t
Liu G, Gaines JC, Robbins KJ and Lazo ND. Kinetic profile of amyloid formation in the presence of an aromatic inhibitor by nuclear magnetic resonance. ACS Medicinal Chemistry Letters 2012, 3, 856-859.
PMID: 24900390 PMCID: PMC4025671 DOI: 10.1021/ml300147m
Liu G, Robbins KJ, Sparks S, Selmani V, Bilides K and Lazo ND. Helix dipole effects in peptide self-assembly to amyloid. Biochemistry 2012, 51, 4167-4174.
PMID: 22559877 DOI: 10.1021/bi3001616
Sparks S, Liu G, Robbins KJ and Lazo ND. Curcumin modulates the self-assembly of the islet amyloid polypeptide by disassembling α-helix. Biochemical Biophysical Research Communications 2012, 422, 551-555.
PMID: 22579683 DOI: 10.1016/j.bbrc.2012.05.013
Robbins KJ, Liu G, Lin G and Lazo ND. Detection of strongly bound thioflavin T species in amyloid fibrils by ligand-detected 1H NMR. Journal of Physical Chemistry Letters 2011, 2, 735-740.
Liu G, Prabhakar A, Aucoin D, Simon M, Sparks S, Robbins KJ, Sheen, Petty SA and Lazo ND. Mechanistic studies of peptide self-assembly: Transient α-helices to stable β-sheets. Journal of the Americal Chemical Society 2010, 132, 18223-18232.
PMID: 21138275 DOI: 10.1021/ja1069882