Analysis of centrosome associated proteins in Dictyostelium
Our long-standing interest in cytokinesis has led us to explore the roles of a number of genes/proteins involved in this process. Recently, we have turned our attention to centrosome-associated proteins, proteins involved in vesicle trafficking, and components of the SIN/MEN pathways. Centrosomes are the main microtubule-organizing centers (MTOC) of most animal cells (Doxsey et al., 2005). They govern the organization of microtubules that are used as tracks for vesicle and organelle transport, cytoskeletal support, and the generation of the spindle and astral microtubules during cell division. Centrosomal defects have been linked to genetic instability (Pihan et al., 1998), chromosome missegregation, and aneuploidy (Brinkley, 2001), all factors that are widely accepted as hallmarks of various cancers. In fact, centrosomal dysfunctions have been observed in most types of cancer (Lingle and Salisbury, 2000; Doxsey, 2001; Giehl et al., 2005). Centrosome abnormalities have also been linked to neurological diseases. Supernumerary centrosomes have been observed in fibroblast cell lines derived from Huntington’s disease patients (Sathasivam et al., 2001) and the Parkinson’s disease- related protein parkin localizes to the centrosome under certain conditions (Zhao et al., 2003).
Importantly, centrosomes have also been shown to play a role in cytokinesis. The removal of centrosomes by laser ablation (Khodjakov and Reider, 2001) or microsurgery (Hinchcliffe et al., 2001; Piel et al., 2001) resulted in failure at the latest stage of cytokinesis, or cell abscission. Our underlying goal has been to further develop Dictyostelium as a model system for the study of the centrosome and its role in cytokinesis. We have begun by first targeting a number of gene products by RNAi technology (see below) and assessing the effects of such “knock downs” on cell functions. Included in our preliminary list of gene targets are the kinases polo-like kinase (PLK) and Aurora kinase (AurK), Sun1, a potential anchoring protein, Sec15 and Exo70, members of the exocyst complex, and Bub2, a member of the SIN/MEN pathways.
DdCenB, a novel centrin
Centrins are a family of small, calcium-binding, calmodulin-related proteins that are typically associated with centrosomes and centrioles (Schiebel and Bornens, 1995). In fact, yeast centrin, Cdc31p was the first centrosomal component to the cloned and sequenced (Baum et al., 1986). Centrins are found in all eukaryotes and their high degree of conservation suggests that these proteins are essential for cell function. For example, conditional mutations in Cdc31 results in cell cycle arrest and failure to duplicate the yeast spindle pole body (Ivanovska and Rose, 2001). In mammalian cells the reduction of centrin protein by RNAi results in cell division arrest and cytokinesis failure (Salisbury et al., 2002). A mutation in Chlamydomonas centrin induces chromosome loss and genomic instability (Zamora and Marshall, 2005). More generally, roles in centrosome/SPB duplication and cell division have emerged for these proteins. For example, the injection of heterologous centrins (centrin isolated from Chlamydomonas or human centrin2) into Xenopus embryos was able to dramatically impair cleavage in the injected blastomeres through a dominant negative effect (Paoletti et al., 1996).
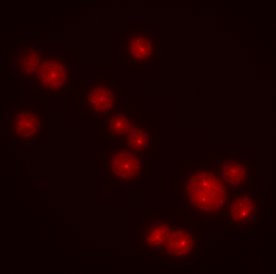
Lower eukaryotes typically have a single centrin gene, whereas higher eukaryotes, including humans, have four distinct centrin genes. In Dictyostelium a divergent member of this family had previously been described (DdCrp – Dictyostelium discoideum centrin- related protein) (Daunderer et al., 2001). DdCrp localizes to the centrosome and this localization is cell cycle-dependent, as it disappears from the centrosome during prometaphase. DdCrp was also found in the nucleus but this localization did not change through the cell cycle. More recently, a second centrin-related protein was identified through the Dictyostelium genome sequencing project and has been named DdCenB (Graf et al., 2004) (DdCrp has since been renamed DdCenA). In order to study DdCenB we have cloned and expressed the coding sequence for this protein behind the red fluorescent protein (RFP). Interestingly, we found no evidence for centrosomal localization of DdCenB-RFP in whole cells or isolated centrosomes. Instead, DdCenB localized to the nuclei of interphase cells. Localization disappeared as cells entered mitosis, even though Dictyostelium cells undergo a closed mitosis in which the nuclear envelope does not breakdown. Our goal is to elucidate the function of DdCenB, with a watchful eye on its role in cell division.
Using Dictyostelium to study a harmful parasite
In 2005, Song et al, published data comparing the genomes of several model organisms, including those of two Amoebozoan species: the human pathogen Entamoeba histolytica and Dictyostelium discoideum. Seventy-eight common and unique genes were identified and can thus be viewed as prospective targets for the development of new anti-amoebic drugs. Due to the pathogenicity of Entamoeba, studying these genes in the organism itself poses a significant health hazard to the researcher. Therefore, the closely related Dictyostelium can alternatively be utilized to study these target genes. The goal of this project is to exploit the experimental flexibility of Dictyostelium as a useful model to study the functions of these unique genes. As a first approach, RNA interference was used to knock down the expression of six of the 78 unique genes, with subsequent molecular and phenotypic analyses. As a second method for acquiring insight into gene function, PCR based gene knockout constructs were created, targeting two Dictyostelium genes, DDB0187415 and DDB0168763. These analyses are currently underway.