The regulation of enzyme catalysis and protein function resulting from the specific interactions between two or more proteins plays an integral role in cell division and homeostasis. The development of various human diseases can occur when these protein-protein interactions are disrupted or modified. Understanding how these protein-protein interactions affect an enzyme’s ability to make or break bonds and its underlying mechanism is an important step in drug design/development.
Ubiquitylation involves the transfer of the small 76-residue protein ubiquitin between a series of enzymes (E1, E2, E3) until it labels the ε-amino group of a lysine residue on a substrate protein. This pathway is involved in many different intracellular processes including protein turnover, cell cycle progression, signal transduction, DNA repair and transcriptional regulation.
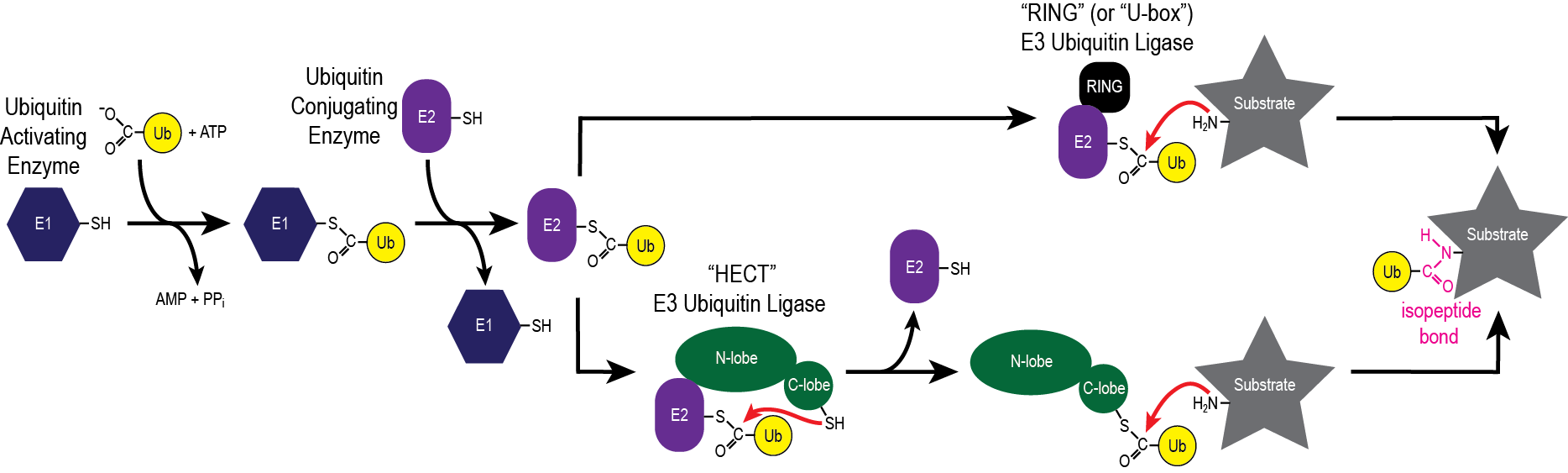 Our research program focuses on deciphering the unique mechanisms employed by members of the HECT (Homologous to E6AP Carboxyl Terminus) E3 ubiquitin ligase family. Working at the interface of physical biochemistry and chemical biology, our research team uses biochemical and biophysical techniques including NMR spectroscopy to understand how HECT E3 ubiquitin ligases attach ubiquitin or ubiquitin-like proteins to a substrate protein. Specifically, our group is focused on the following themes:
Our research program focuses on deciphering the unique mechanisms employed by members of the HECT (Homologous to E6AP Carboxyl Terminus) E3 ubiquitin ligase family. Working at the interface of physical biochemistry and chemical biology, our research team uses biochemical and biophysical techniques including NMR spectroscopy to understand how HECT E3 ubiquitin ligases attach ubiquitin or ubiquitin-like proteins to a substrate protein. Specifically, our group is focused on the following themes:
- Molecular basis for specificity in ubiquitin chain linkage by the HECT E3 ligases
- Mechanism and ubiquitin handling by the HECT E3 ligases
- Substrate recognition by the HECT E3 ligases
Our studies will help to clarify how HECT E3 ligases form multi-protein complexes to build ubiquitin chains and how the malfunction of these complexes cause various human diseases.